siRNAs: From Petunias to Therapies
Over 35 years ago, molecular geneticist Richard Jorgensen set out to create the perfect petunia, one with extremely dark purple petals. To achieve this, he transfected the plants with additional copies of the gene which he knew was responsible for the purple pigment in the flower. Surprisingly, the resulting flowers did not have the expected dark purple colour but rather had no pigment at all.
A similar observation was made years later by researchers Andrew Fire and Craig Mello. They injected the roundworm Caenorhabditis elegans with double-stranded messenger RNA (mRNA) that encoded various muscle proteins. They observed a twitching effect which is characteristically observed in worms that lacked the gene encoding these muscle proteins. The scientists concluded that the presence of the double-stranded mRNA (a molecule that is not naturally found in the cells) silenced the gene. These findings eventually led to the award of the Nobel Prize in 2006 for the discovery of small interfering RNAs.
Small interfering RNAs (siRNAs) are part of a natural defence mechanism that protects against the invasion of foreign genes. It has the potential to silence any gene in a sequence-specific manner. The therapeutic potential for siRNAs for the treatment of genetic diseases was quickly recognised. However, despite significant research efforts in this field, only very few therapies have been approved.
One of the diseases where siRNAs were believed to be a promising treatment option was SOD1-related amyotrophic lateral sclerosis (ALS). The defective SOD1 gene in these patients produces a toxic protein that kills motor neurons, ultimately leading to paralysis and death, usually within two years from diagnosis. As the mutant SOD1 gene is the sole cause of ALS in these patients, researchers reasoned that silencing this gene using siRNAs would result in clinical improvement.
In a recent breakthrough study, it was reported that this strategy does indeed work. Tofersen™ (a drug containing siRNAs directed against the mutated SOD1 gene) led to significant improvements in the patients’ symptoms in a phase III clinical study. Researchers commented that this is, in fact, the first trial in which patients have reported an improvement in their motor function. Whilst Tofersen™ is only effective in the small proportion of patients having the SOD1 mutation, this nevertheless provides an important breakthrough and gives hope for patients suffering from other forms of ASL and also other diseases caused by gene mutations. Indeed, recent years have seen the regulatory approval of other siRNA drugs which treat metabolic diseases like hereditary transthyretin amyloidosis, acute hepatic porphyria and primary hyperoxaluria type 1. Many more siRNA drugs are in development for different diseases, including neurological disorders, inflammatory disorders and cancer.
Whilst the underlying concept of siRNA therapy seems simple, the practical implementation has proven difficult and has required substantive innovation. A major problem is that RNA molecules are unstable and prone to degradation. Furthermore, only a few cell types in the body are able to take up naked siRNA. Thus, arguably, the biggest obstacle was the delivery of the siRNAs to the target cells without metabolic clearance and immunogenicity. In fact, some sources attribute Tofersen’s initial failure in clinical trials to poor delivery to the brain.
For certain tissues like the eye, skin, or mucus membranes, siRNAs can be delivered topically or locally. Where local delivery is not an option, the siRNAs need to be “packaged” in order to be delivered to the target tissue. Nanoparticles are most commonly used for that purpose. They protect the siRNAs from degradation and can be engineered with target-specific ligands so that the siRNAs are delivered to the correct target cells.
A lot of innovation has also gone into the development of optimised siRNAs. These should show high specificity for the target mRNA with no or few off-target or toxic effects, can efficiently cleave the target, have a long half-life in the body and not trigger the innate immune response. Modifications that have been made to achieve this such as optimisation of the length of the siRNAs, variation of the “GC content” (i.e. composition of bases that make up the siRNA), or avoidance of secondary structures. Much research has also gone into chemical modifications that improve chemical stability and reduce immune reactions. These modifications have been found to be useful in the various parts of the siRNAs i.e. the ribose moiety, the phosphate backbone, and the base.
The significant innovation that has gone into the development of siRNA therapies is reflected in the number of patent applications that have been published:
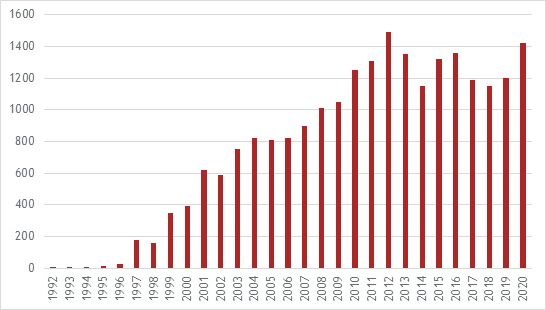
There can be no doubt that continuing innovation will lead to the approval of new drugs resulting in a significant expansion of siRNA therapies in the future.
