The Many Faces of Cell-Free DNA Analysis
“Cell free DNA” (cfDNA) refers to fragmented extracellular DNA that is present in bodily fluids of a subject, most notably in blood.
The origins of cfDNA are diverse, but the bulk of cfDNA typically derives from the everyday turnover of cells, which involves the fragmentation and release of genomic DNA. In addition to routine cellular turnover, there are multiple types of “non-self” sources of cfDNA including: (i) cancer cells; (ii) fetal cells; (iii) transplanted organs; and (iv) infection.
The analysis of the make-up of a patient’s cfDNA profile can therefore provide a wealth of information about these “non-self” sources. cfDNA analysis has been employed in a wide range of applications including cancer diagnosis and monitoring, prenatal diagnosis, monitoring graft rejection in transplant patients, and diagnosis of infectious diseases. All of these applications obtain their analyte through a simple blood draw. In part due to this minimal invasiveness, methods of analysing cfDNA populations have received great attention over the past decade and are quickly becoming a key component in global healthcare.
The emergence of cfDNA analysis
The existence of cfDNA, however, is not a recent discovery, with cfDNA being detected as early as 1948. It has, however, not been until the last decade or so that the necessary technologies have been available to analyse cfDNA at the accuracy and throughput required to enable commercialisation of cfDNA profiling.
A significant contributing factor to the emergence of cfDNA as a diagnostic tool has been the advances made in the field of next generation sequencing. These advances have allowed for the in-depth cfDNA analysis at a single nucleotide resolution and in an economically-viably manner. In particular, the reduction in cost associated with sequencing has helped bring cfDNA-based assays into the clinic.
cfDNA profiling in cancer
One of the most widely adopted applications of cfDNA analysis is in cancer detection and monitoring. As with the death of normal cells, tumour cell death can result in the release of cfDNA fragments into the bloodstream. These tumour-derived cfDNA fragments (also termed ctDNA) can have distinctive signatures, providing valuable information about its originating cell. Such signatures can be in the form of somatic variants, methylation status and/or fragmentation patterns, all of which have been shown to differ in ctDNA relative to non-cancerous cfDNA.
Moreover, the analysis of ctDNA profiles can also provide an insight into tumour heterogeneity through determining the relative abundances of different somatic mutations. This is an important advantage over tissue biopsy-based assays because, unlike tissue biopsies, the tumour cells that contribute to the ctDNA profile are not confined to a localised portion of the tumour, and thus are more representative of the tumour as a whole. The monitoring of tumour heterogeneity over time can, in turn, be used to observe the evolution of the tumour and the behaviour of different clonal populations, such as the eradication of one clonal population in response to a particular therapeutic, but the emergence of a new, drug-resistance population. ctDNA analysis in combination with targeted treatments, therefore, opens the door to a highly personalised approach to cancer therapy.
The use of ctDNA analysis for therapy selection in cancer patients has already become a reality, with the Guardant360® CDx test being approved by the FDA in 2020. This test provides somatic mutation analysis of cfDNA and can be used to guide treatment selection in cancer patients. The routine use of cfDNA analysis for the early detection of cancer is also coming closer to a reality. In 2021, NHS England announced a 140,000 patient trial of the Galleri® blood test, which aims to detect more than 50 types of cancer via the analysis of cfDNA methylation patterns.
The potential opportunities in this field are clearly massive, from both a public health and economic perspective. For example, 57% of people with lung cancer survive their disease for 5 years or more when diagnosed at stage I, compared with only 3% of those diagnosed at stage IV, emphasizing the importance of early detection. Integrating cfDNA-based assays into routine health check-ups would detect cancer earlier, ultimately improving patient survival rates. From an economic standpoint, the global liquid biopsy market is expected to hit $26.2 billion by 2030, compared to $7.1 billion in 2020.
cfDNA profiling in prenatal diagnostics
Another field where cfDNA analysis has gained significant traction in the last decade is prenatal diagnostics. In 1997, Dennis Lo demonstrated that cfDNA derived from the fetus was present in maternal blood. Since then, the analysis of the cell‑free fetal DNA (cffDNA) has become a valuable tool for prenatal diagnosis. Similar to cfDNA profiling for cancer detection, the analyte is obtained through a simple blood draw, and the analysis of cffDNA has thus been termed non-invasive prenatal testing (NIPT).
To date, NIPT has primarily been used for fetal screening for aneuplodies, particularly trisomies of chromosomes 21 (Down’s syndrome), 18 (Edwards’ syndrome), and 13 (Patau syndrome). Traditionally, these trisomies were identified through either amniocentesis or chorionic villus sampling, but there is an increased risk of miscarriage associated with the invasive nature of both of these techniques. NIPT has therefore been favoured as a method of providing an insight into the presence or absence of these trisomies, without the associated risk of misscarriage.
In 2018, it was estimated that over 10 million NIPT tests were performed worldwide, with the global market size expected to grow from ~$3.5 billion in 2020 to ~$13 billion by 2028. Similar to cancer detection, it seems that cfDNA analysis is set to play a significant role, if not the dominant role, in prenatal testing for the foreseeable future.
Outlook
As with any technologies with such a large market potential, there is a huge number of both start-up and established companies innovating in this hotly contested area. This is demonstrated by the striking increase in the number of patent applications published relating to cfDNA analysis since 2010, as shown in the chart below.
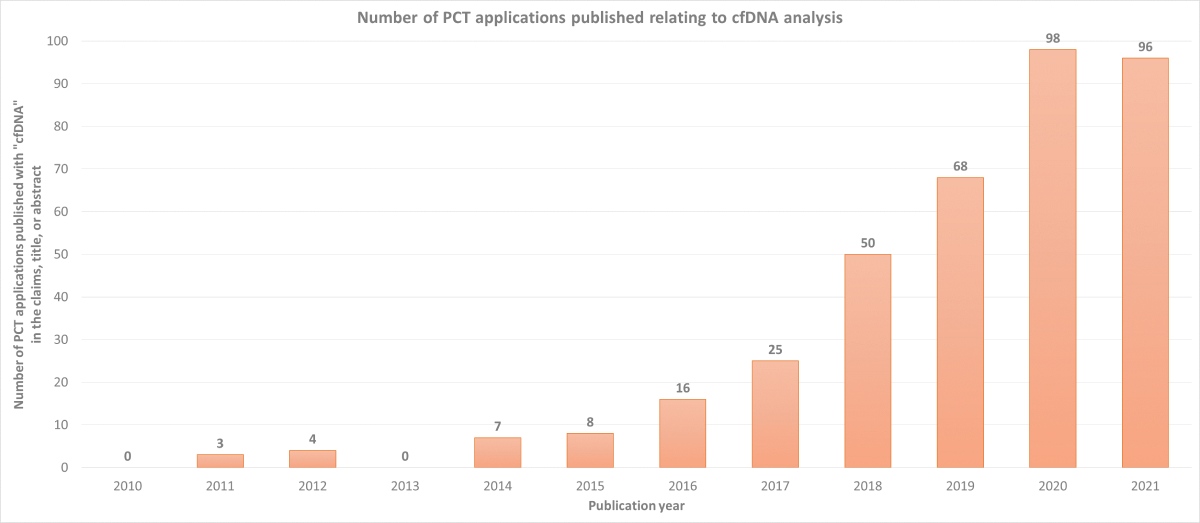
Such innovation will undoubtedly continue to advance the effectiveness and utility of cfDNA‑based assays, cementing their position in mainstream healthcare for the foreseeable future.
