Organocatalysis: Reflecting on Two Decades of Innovation
Catalysis open the doors of chemistry and provides us with greater access to the natural resources in the world around us. Specialised molecules (catalysts) can offer certain chemical reactions lower energy, faster pathways towards their products, all while not being consumed in the process. In the humanised world of chemistry, catalysts are the motorways, allowing a faster journey to the destination, albeit at the cost of their initial construction.
2021 saw the Nobel prize in chemistry awarded for the development of organocatalysis, a subsection of the field focussed on the use of simple organic molecules. As is often the case with these awards, the recognition of the discoveries and hard work came as a relief to all those involved. In a science as diverse and complicated as chemistry, it is important to take the time to reflect upon the achievements of the last couple decades and to consider where it could eventually lead us.
Organocatalysis: An Abridged History
In 1746, a British industrialist named John Roebuck made chemical history with the first recorded use of industrial catalysis. Lining reaction chambers with a thin layer of lead, Roebuck was able to catalyse the reaction between steam, sulfur dioxide and nitrogen dioxide to produce sulfuric acid on an industrial scale.
In the many centuries since, the use of catalysis in an industrial setting has boomed, and the catalyst market has snowballed in size, valued globally at $37 billion in 2021 and set to sky rocket to around $61 billion by 2030.
With around 90% of industrial chemical processes reliant on catalysis, its application is essentially endless and includes exceptionally important processes such as the cracking of crude oil, scrubbing of flue gases and the function of catalytic converters.
However, as the world becomes increasingly conscious of the new challenges we face in the twenty-first century, our eyes can’t help but be drawn to some of the limitations of traditional catalysis. The vast majority of catalysts employed in the industry today are sourced from non-renewable, difficult to recover and highly toxic metals. With the rising costs of these elements and diminishing supplies, alternative approaches to traditional metal-catalysis are becoming increasingly popular. It appears that this demand for a ‘green’ alternative can be met in many cases through the use of enzymes and organocatalysis.
While enzymes are essentially nature’s answer to catalytic processes, organocatalysts strip away the protein backbone, leaving what is essentially a naked ‘active site’. This results in (usually) very simple organic molecules that are easy to manufacture and that can have an exceptionally high catalytic activity.
Before its research boomed in the early 2000s, there were few examples of organocatalysis in the literature, and even fewer of the more desirable asymmetric variations. The most famous of these was arguably the Hajos-Parrish-Eder-Sauer-Weichert (HPESW) reaction, a mouthful of a reaction discovered in the ‘70s and regarded at the time as much of a novelty. The HPESW reaction made use of the amino acid (S)-proline to catalyse an asymmetric aldol reaction. The untapped brilliance of reaction was the ability for a molecule as small and simple as proline to catalytically introduce high levels of asymmetry in its reaction products.
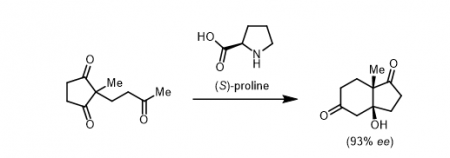
The Hajos-Parrish-Eder-Sauer-Weichert reaction
A Nobel Prize winning field
Building on this understanding, and with dreams of catalysing many reactions using small organic molecules found in any laboratory, Benjamin List and David MacMillan independently began researching this idea in the early 2000s. List began by investigating the use of proline as a catalyst for asymmetric intermolecular aldol reactions (more challenging and wider-scoping variants of the HPESW reaction). Simultaneously, MacMillan began investigating the use of iminium ions as a means of activating enones for selective Diels-Alder reactions.
Both List and MacMillan ultimately succeeded in what they had set out to achieve: the use of small organic molecules to catalyse reactions with impressive selectivity. By sheer coincidence, the two submitted their discoveries to the Journal of the American Chemical Society within very short succession, and they were both awarded the 2021 Nobel Prize in chemistry for their pioneering work in the field.
Where will it take us?
In the years since its initial recognition, organocatalysis has been the focal point of many independent researchers. This has resulted in a large number of patent applications being filed, including those claiming their use for pharmaceutical and polymer synthesis. The simplicity of the organic molecules has, in many ways, allowed the chemist to be more creative in their design and approach than traditional metal-catalysis may allow. Ideas have ranged from the use of bizarre oxidation states of sulfur, iodine and phosphorus through to the synthesis of so called organic ‘superbases’.
The end result is a broad and diverse field with links to other rapidly developing technologies, such as flow chemistry, photochemistry and organoredox chemistry. This has doubled-down on many reactions ‘greenness’, improving aspects such as catalyst recovery and allowing for a reduced solvent dependency.
One creative solution towards catalyst recovery is provided by US 20130096334 and involves the coupling of organocatalytic molecules to magnetic ferrous nanoparticles. The use of higher-toxicity metals can be avoided and the catalyst separation can be aided by passing the reaction composition through a magnetic field.
The low-toxicity and low-cost of organocatalysis may eventually make the technology common place in our day-to-day lives. A new organocatalytic battery additive has been developed that will slowly coat its electrodes in a protective film (CA 3019601). This minimises side reactions with trace water and allows for an improved battery stability.
The beautiful simplicity of organocatalysis has far from restricted the creativity of the chemists involved nor its implementation in widespread applications. The dawn of organocatalysis may have been at the turn of the century, but many are optimistic that a golden age is still to come.
J A Kemp has expertise in this fascinating technology. For more information see our Chemistry; Industrial and Chemical Processing; Pharmaceuticals; and Polymers specialisms.
